Harrow, Inc.: Cleared vision?
[The shareholder letters and earnings reports can be found on Harrow’s website: www.harrow.com. The transcripts of the earnings calls are provided by various financial services.]
For my last article on Harrow I received some push-back from individuals bullish on the stock. One comment I got was that I was a (very unsophisticated) retail short-seller desperately trying to bring people to sell by floating exaggerated doubts and feeble arguments on the Internet.
So perhaps first of all I should put the pronounced bearishness of my last article into perspective.
In writing it, my intention was to see: from here, after Q3 2023 that disappointed the market so much, what is the floor? That's why in the article, I said: let's assume Harrow hit peak revenue in Q3 2023. This entails that the compounding business, the Fab 4 and Iheezo will flatline from here onwards, that Vevye will be outcompeted by Miebo, that Triesence will fail commercially and only rake in enough to cover the added cost of debt. And the $7m cash-burn annually will continue unabated. But even in this scenario, when everything goes wrong for Harrow, I concluded they have 7 more years to breathe — without issuing equity. And after 7 years, given their track record, they should have sorted things out. What I found odd, though, was the CEO Mr. Baum giving guidance for 2024 about remaining confident to be able to service debt, when, according to my view, this should have been a non-issue. So maybe I set the floor wrong, and should have set it even lower; or maybe the wording Mr. Baum used was just not ideal for conveying the idea of financial stability.
Anyways, my article was as bearish as one could get.
So this time, to enlarge the picture, I will present a more bullish view in direct reaction to my bearish comments from before. In this way, the present article will in fact counteract the former one. What this also means is that: I have no opinion on the stock. This blog post represents no investment advice in any shape or form, neither for going long the stock, nor for shorting it.
With the FY 2023 results out since late March, here I’ll review three issues I highlighted before: accounts receivable, the Iheezo chart, and the CMS risk. I’ll also take a look at a new accounting item that initially caused some headache to shareholders: a modified version of accounts payable. And at the end, I’ll draw a tentative bull case for 2024 (not to be taken seriously and not be acted upon, of course; see the disclaimer above). So here we go.
Accounts receivable
While me wondering suggestively about in which item of Harrow’s income statement uncollectible accounts receivable (AR) might be hidden was, admittedly, a bit over the top, the general concern about ballooning AR appears to have been legitimate, and is legitimate still.
On the Q3 2023 earnings call the CFO Andrew Boll anticipated collecting over $16m in AR from the branded products in Q4, in addition to the AR from the compounding business, and expected more cash inflow and a smaller AR to revenue ratio going forward. Accordingly, in my last article I wrote: “If accounts receivable don’t drop down substantially, as Mr. Boll affirms they will, it will cast further doubt either on management’s ability to assess the progress of their business, or on their honesty vis-à-vis their shareholders.”
Now, indeed, AR did not scale back in Q4. The exact opposite happened: AR shot up sharply.
Here’s the weight of AR on the asset-side of the balance sheet at the end of Q3 2023:
And here it is at the end of Q4 2023:
The ballooning accounts receivable ballooned even more, at an unprecedented pace. At the end of Q3, they stood at $18,468,000 on the asset side of the balance sheet, having risen by $12,287,000 in the nine preceding months. But then, just in three months, in the fourth quarter of 2023, AR almost doubled from that to $36,261,000. For the whole year, Harrow’s AR surged by $30,344,000.
No explanation is given for the doubling of AR in the fourth quarter. Obviously, Mr. Boll’s assessment that the cash collection cycle would normalise in Q4, and hence that the spread between AR and revenue would narrow, was inaccurate. To the contrary, the spread widened to an extent never seen before by the company.
And here’s the interpretation I suggested for the ballooning AR in Q3 2023, and feel inclined to suggest still for Q4:
“Finally, inflated account receivables might be an indication of the practice of overloading the supply chain, also called ‘channel stuffing’. In the case of Harrow, if any of the scenarios presented here is true, I estimate this to be the most likely. … To convince distributors to acquire more than their immediate requirements, they are offered significant price reductions, refunds, and extended payment periods. Overloading the supply chain leads to a sudden increase in accounts receivable.”
Still, it is possible that Harrow, as anticipated by Mr. Boll, collected the $16m and some, and it was substituted by new AR created by strong demand for the drugs and the generously extended payment terms the company offers to customers. In this reading, the surging AR, rather than indicating channel stuffing, would indicate continued, tremendous growth.
This very positive reading, however, is hard to reconcile with what we know: that Iheezo sales dropped markedly in Q1 2024.
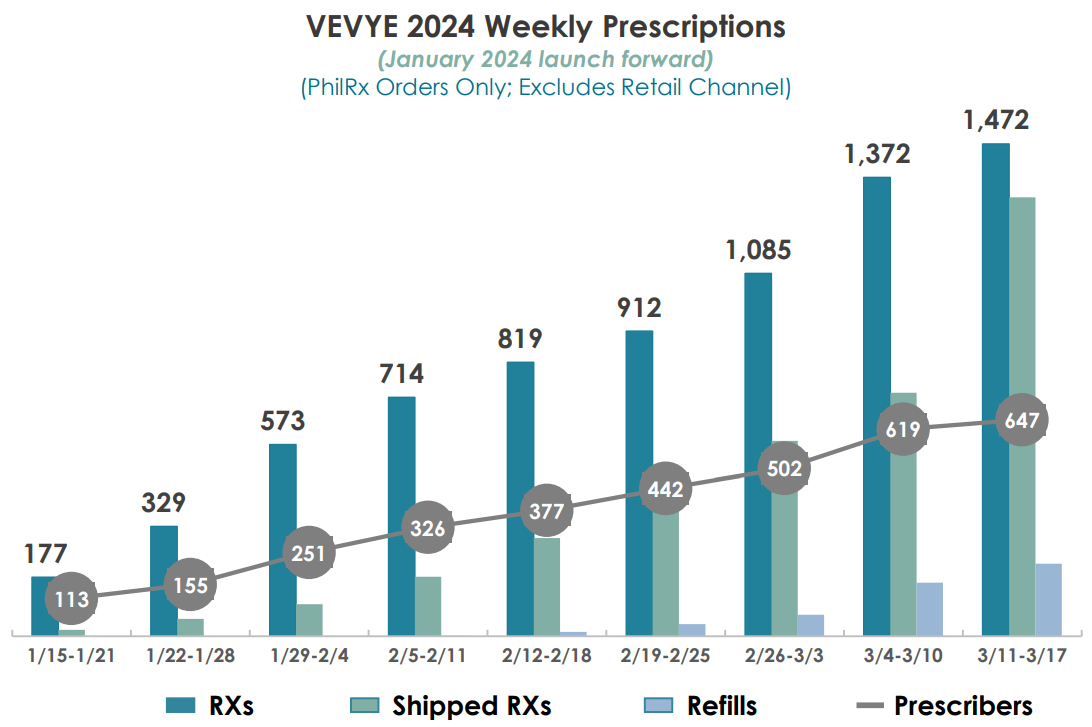
The Iheezo chart
In the section of my last article which I called “The imperfect chart crime”, I argued that from the chart management printed to display Iheezo’s success not much insight could be drawn about Iheezo’s success: “The choice of the data points – 14 April, 12 May, 4 August and 27 October – is puzzling, and indeed no reason is given for their use.” Besides, the chart tracked “trailing twelve week customer unit demand”, a construct that further clouded the perspective on how the Iheezo sales were progressing.
I’m aware I was not the only one who found the chart confusing, and thankfully management provided a far more readable one in the Q4 2023 presentation:
Of course, “unit demand” is neither revenue nor profit, so it does not tell us how promotional Harrow had to be with discounts to fuel the demand, or if it was fueled by Iheezo just being an amazing drug of its own.
Apart from this, and most importantly, in the chart above we see no progression of the sales into Q1 of 2024.
Mr. Baum revealed in the FY 2023 letter to shareholders that in Q1 2024 the sales of Iheezo have been and continue to be affected by a digital security breach at Change Healthcare, a medical claim processing company. As a result, Change Healthcare has been unable to handle medical claims by Harrow’s customers. Therefore, the customers, with less cash in their revenue cycle, have ordered less from Harrow, affecting the latter’s sales figures.
But to be clear: The cyber attack didn’t occur before the 21st of February, so it doesn’t account for the absence of Iheezo figures in the presentation up to the time the cyber attack occurred.
To generously explain away the absence of 2004 Iheezo numbers in the FY 2023 presentation, one might point out just that: that Harrow was reporting FY 2023, and not Q1 2024. However, for its other new flagship drug ‘Vevye’ Harrow did not fail to include numbers for 2024 in the FY 2023 presentation:
Why this diverse treatment? The likely reason is: that in Q1 2024, Iheezo demand did not continue to go up and to the right. Else, management would not have failed to let shareholders in on the good news, like they let them in on the good Vevye news.
Even the Harrow bulls admit that the significant surge in Iheezo sales in December was primarily influenced by an end-of-year stocking effect. This conclusion relies on — albeit incomplete — Bloomberg institutional data, according to which sales levels substantially declined in January and then slightly rose again in February. Corroborating this, an individual investor claims to have read a report presenting Iheezo sales for Dec/Jan/Feb as follows: $5m/$1m/$2m. While I have no way of validating this claim, the numbers are plausible and in accordance with Bloomberg data.
A possible explanation for the downward move in sales is that some of the incentives offered to distributors for stocking Iheezo expired as the calendar year ended. Harrow provides an extended payment window of 30 or 45 days. Consequently, distributors could secure rebates by ordering more stock than usual, even if it meant bloating their inventory. Since payment is deferred, there’s no reason not to take advantage of such an end-of-year opportunity. Whether this specific scenario of year-end incentives or another one accounted for it, there indeed was a stocking effect in December. As a result, excess inventory remained on distributors’ shelves going into January and February, leading to lower order volumes while this surplus inventory kept unwinding.
This, by the way, is the scenario I outlined in my last article, as mentioned above; I just used a different word. If you believe that ‘stocking effect’ is a more urbane term than ‘channel stuffing’ (which I employed), then I tend to agree with that, but the underlying reality remains the same. Consequently, Iheezo sales had to fall off a cliff somewhen; as I wrote: “It [channel stuffing] can inflate revenue figures for some months, as it heavily frontloads future transactions.” And the falling off the cliff happened sooner than I expected, already in January. And for January Harrow’s management failed to show the Iheezo data, while they did not fail to show the flattering Vevye data.
Greater transparency on the part of Harrow's management would be desirable. Mr. Baum frequently claims to follow the ideal of Berkshire Hathaway in building a company built to last, on the principles of transparency and integrity. This should entail not just showcasing the successes to shareholders, but also discussing the shortfalls, even if they should turn out to only have been temporary. This under-communication by very selective communication, is also reflected in the communication about operating cash flow for FY 2023.
Accounts payable
In presenting the FY 2023 results, Mr. Baum stressed that Harrow was positive in operating cash flow for the year. It was the last quarter that buoyed Harrow out on that front: “And finally, [we achieved] delivering $8.7 million in cash from operating activities in the fourth quarter of last year and delivering our second consecutive year of positive cash flow from operations” (FY 2023 earnings call).
But Harrow being cash flow positive on the year was just due to one fact: they put off paying their bills. As Harrow still has to pay them, but hasn’t paid them yet, their cash flow is temporarily increased.
Here is what accounts payable looked like on the balance sheet at the end of Q3 2023:
And here is what they looked like one quarter later, at the end of Q4 2023:
Note that, first, accounts payable roughly doubled, from ca. $13m to ca. $25m. But moreover, a new item appeared, not present before: “Accrued rebates and copay assistance”.
This new item is also reflected in the cash flow statement. At the end of Q3 2023, the cash flow was increased by “accounts payable and accrued expenses” to the tune of $1,584,000:
That same item, now enlarged by “accrued rebates and copay assistance”, surged to $31,795,000 (!) at the end of Q4 2023:
Hence, what drove operating cash flow in Q4, and made Harrow finish operating cash flow positive for FY 2023, was simply the change in “Accounts Payable”.
That's it. That's the item that made the difference, and enabled Mr. Baum to achieve a cash flow positive number to sell as a success to shareholders.
According to Harrow’s information to individual shareholders, the new item on the balance sheet and cash flow statement is a result of the termination of profit sharing that before Harrow had for some drugs with Novartis and Santen. Now Harrow has to break out the rebates and co-pay that before Novartis and Santen did. This new item is also supposed to include the rebates and co-pay for Iheezo in Q4, and will include them for all branded products going forward. Hence, while revenue is being recorded net of rebates and co-pay, the latter two have to be recorded as a separate item in the accounting, and this is what happened in the Q4 and FY 2023 reporting.
Although this clarification explains this puzzling new item, I would like to point out that I wouldn’t feel inclined to sell a positive cash flow as a success when that cash flow was only positive because I haven’t yet paid the bills (in form of rebates and co-pay) I will need to pay soon. A strong positive cash flow, to the contrary, would be a cash flow positive before changes in assets and liabilities, as with changes in the latter the underlying cash flow picture of the company can appear distorted to some extent.
And, again, while the new item has been explained by Harrow to individual shareholders upon request, I wonder why management didn’t explain it already on the earnings call, since solely thanks to that item there was the positive cash flow that management didn’t miss out on highlighting prominently.
CMS / Iheezo in-office
Iheezo — an ophthalmic gel for ocular surface anesthesia — is used both in hospitals and in-office settings, and the latter are of paramount importance for Iheezo becoming the success management wishes it to be.
Iheezo has a permanent J-Code for the office setting, which means that doctors using it there get reimbursed for the product by Medicare. Management announced on the Q2 2023 earnings call that doctors in-office had been briefed on how to ‘build’ the J-Code and that they were getting reimbursed for Iheezo. With that, no barriers appeared to be left to the widespread adoption of Harrow’s flagship drug.
But suddenly, in Q3 2023, a new issue came up, included in the “Risks” section of the Q3 report. It pertained to the CMS billing policy. CMS, the Centers for Medicare & Medicaid Services, is the federal agency that administers the nation’s major healthcare programs, including Medicare. The “Risks”-section stated that, if CMS didn’t agree with Harrow’s view, doctors in-office might either bill for the anesthesia/drug itself (that is, Iheezo) or for the service of providing anesthesia, but not for both.
Accordingly, if CMS had disagreed with Harrow, the enticement for doctors to use Iheezo in-office would have been considerably lower, because they would have been either reimbursed for Iheezo or for the service of them administering Iheezo, but not for both.
In Q3 management didn’t disclose which impact on their expected revenue from Iheezo the possibility of CMS not agreeing with them would have, and even sounded an upbeat note about the upcoming meeting with CMS, making the matter appear as hardly relevant to Iheezo’s success: “That said, we are selling into that market and reimbursements are happening. And this, I think will depending on the outcome of the meeting, I think have a significant upward potential value impact on that asset and that’s why we’re going to be meeting with CMS. So once again, we don’t control that decision-making, but we’re excited to meet with CMS in the fairly near term” (Q3 2023 earnings call).
Much to the delight of Harrow’s management and its shareholders, surrounding the FY 2023 reporting, on the CMS front only good things happened.
On March 19 2024, Harrow received confirmation from CMS that Iheezo is separately payable in the physician office setting.
Mr. Baum didn’t spare words about the significance of what the positive decision of CMS meant for Harrow: “The one comment that I'll make is what I think is the 800-pound animal in the room, [...] the confirmation from CMS that in fact, IHEEZO will be paid for separately in the physician's office setting is, without a question the most positive consequential event, I think, that has happened to our company since I founded it with Andrew back in 2011. So this is a very big event.”
The news represented such a consequential development that at Harrow prayers were being said for it: “Indeed, yesterday was special for every member of the Harrow family, and it was an answer to some of our prayers actually.”
Moreover, one day later, CMS granted a related request, that two Iheezo vials could be used and billed per procedure, as many ophthalmologists perform bilateral ocular procedures.
When the founder CEO of the company says that CMS clarifying Iheezo reimbursement is the most important event since the company was founded, then I would presume that if CMS had not clarified the situation according to Harrow’s view, this would have posed a considerable risk to the ongoing success of Harrow as a growth company. But this risk only popped up for the first time in the risk section of the Q3 2023 reporting, and from the wording there as in the corresponding shareholder letter, it wasn’t all that clear what the issue was, and what kind of risk it actually represented. As I noted in my last article: “The shareholders are left in the dark about the possible adverse revenue impact of that risk.” I imagine many a shareholder was surprised that CMS deciding in Harrow’s favour on the matter was so momentous for the future of the Harrow story that it amounted to the best thing ever that happened to the company and that management had to run prayer sessions for it.
Again, this demonstrates, as I view it, a pattern of management over-highlighting the bright prospects of the company, whilst under-reporting the gritty problems it naturally runs into. And again, this would be half as bad if management did not lay a claim to Buffett-like integrity in dealing with shareholders.
According to the reading of the Harrow bulls, though, who are wont to cut management a lot of slack, there never was risk. The CMS was notified about the J-Code application, and the reimbursement process was managed internally. The bullish reading says that Harrow had just difficulty marketing to large buyers because these buyers wanted assurance that the reimbursement policy by CMS was deliberate. Hence, there was a low likelihood of any negative outcome. The bulls point out that the stellar performance of Iheezo happened prior to the CMS update. Hence, the real question was whether an extraordinarily positive event would occur, which could lead to a boost in Iheezo’s revenue, possibly beginning by Q2 2024. And it did occur.
And already having lent the voice to the bulls, this brings us to the Harrow bull case for 2024 I outline in the following; though I’ll temper it with a dose of cautiousness.
The cautious bull case
If we look past the inventory unwinding, Iheezo is likely operating at a $40m annual revenue pace currently. Assuming conservatively an incremental increase only, Iheezo’s revenue could reach $50m in 2024.
The compounding business, Fab 4 and the Santen portfolio combined are projected to generate about $140m this year. Attempts to revive the Fab 4, although management called them promising in Q3 2023, have not brought results so far — and, sure enough, management preferred not to mention them on the FY 2023 earnings call —, thus no increase shall be assumed here. As to the compounding business, which was Harrow’s former DNA before moving into branded products, it has fallen back a little lately (from the FY 2023 presentation):
But management expects that it will return to growth to the tune of 10% in 2024. With this estimate they must already have taken into account that Klarity-C, the compounded product for dry eye disease (DED), will probably lose market share to Vevye, the new branded drug for DED. Klarity-C has likely more than 20k prescriptions per month without a dedicated sales team. Vevye, to the contrary, has a dedicated sales team and is supposed to be the better product, hence, loss in prescriptions to Klarity-C would be overcompensated by higher Vevye prescriptions, even without insurance coverage.
It is worthwhile to dwell a moment on Vevye. There are estimates that about 90% of patients diagnosed with moderate to severe DED ultimately opt not to treat it due to the unpleasant side effects and slow effectiveness of the branded drugs available. For instance, around 90% of ‘Restasis’ users discontinued use after four months because the benefits were too low in comparison to the side effects like burning sensation. Yet, Restasis raked in more than $1.5b annually before its patent expired. This highlights the great potential of this market that Vevye could tap into. Vevye is a better drug than Restasis, with better effectiveness and less side effects. It is one of two new superior drugs for DED — the other one being ‘Miebo’ by Bausch & Lomb — that could potentially divide the market between themselves in the USA.
But the Vevye story is much more a story about what the future holds than one for 2024. For 2024 its contribution might be around $15m, assuming that without insurance support it continues in an initial growth phase.
Combining the estimates from above, we get a total of $205m in revenue, excluding ‘Triesence’ that Harrow hasn’t been able to produce yet.
If Triesence begins production in late 2024, it could contribute $20m. Mr. Baum mentioned that the first three commercial batches would yield 90k units. Priced at $944 per unit, given its J-Code status and high demand, the net revenue for this drug is expected to be substantial. Triesence doesn’t require price reductions to gain acceptance; medical professionals are awaiting its availability. As a corollary, there’s virtually no incremental SG&A (selling, general & administrative expenses). So we have 90k x $944 which gives around $85m, and with cost of sales subtracted and a late start in 2024 we very approximately have the $20m net revenue I suggest above.
But let’s not get ahead of ourselves and assume that albeit Mr. Baum’s optimism Triesence won’t happen. After all, Mr. Baum was already very optimistic in Q2 2023 that the first batch of Triesence would go through, but then had to give shareholders notice in Q3 that the batch didn’t match with the quality requirements to commercialise it. So let’s contain the bullishness and keep at $205m revenue, which is in line with Harrow’s guidance of at least $180m revenue for 2024.
Apart from this, compared to 2023, for Harrow overall an increase in SG&A is likely, primarily to support Vevye’s expansion. Already in Q4 2023 Harrow had an annual run-rate of ca. $45m in cost of sales, of ca. $104m in SG&A, and of ca. $13m in R&D (45+104+13=$162m). These numbers are likely to grow, as Mr. Baum already noted in the Q3 shareholder letter that “we expect our operating costs to increase incrementally” in 2024. Still, as this is the bull-case here, and we’ve been conservative in all the preceding assumptions, we have some lee-way for optimism at this point and will just stick with the Q4 2023 run-rate in operating costs. On top of that, we carry over the ca. $21m in debt servicing from 2023 to 2024, as neither an increase nor paying down of debt is planned.
So we have revenue of $205m (ex Triesence), $162m in operating costs, and $21m of debt servicing, that is, (205-162-21=) $22m in earnings before tax (EBT). With ca. $35m shares outstanding, we arrive at (22/35=) $0.63 in EBT per share. At the time of writing this article, Harrow trades at $13.51 per share and hence at roughly 21 times 2024 EBT/share — if this tentative bull case comes to fruition, of course. This is clearly no bargain. At the same time, for a company that grew revenue from 2022 to 2023 at a clip of 47%, and is here projected to grow it by 57% this year, the valuation does not appear excessive in comparison to other growth companies.
We will see what will happen. I just felt that after the FY 2023 results and the CMS news surrounding it, an update to my last article that would paint a brighter picture was called for. Now both sides are laid out, and the future development of the business will decide between them.